| |
Highlights
Bacteria and other microorganisms colonize our digestive tract as soon as we are born. The types of microorganisms vary based on diet and environment. Because an imbalance in the microorganisms in the digestive tract can cause disease, it is important to identify specific microorganisms that inhabit the gut. These researchers used a synthetic gene oscillator called the Repressilator 2.0 to track bacterial growth and better understand growth patterns in the gut of mice with control and inflamed intestines. The system showed that the bacteria from mice with inflammation had repressilators in a wider range of phases compared to bacteria from control mice. This suggests that inflammation changes the gut environment, which results in different growth patterns in the bacteria.
According to recent studies, there are over 2,000 species of
microorganisms
that live inside your
digestive tract,
namely the stomach and intestines. We know that these microorganisms support digestion and overall health, because too much or too little of a given microorganism can result in
inflammatory diseases,
immune
diseases,
allergies,
and more. Scientific understanding of microorganisms in the gut, though, is very limited. New research by Dr. David Riglar, Prof. Pamela Silver, and colleagues provides the foundation for further understanding of microorganisms in the gut by tracking the growth of bacteria passing through the digestive tract.
The Gut Connection
Bacteria and other microorganisms begin to colonize our digestive tract as soon as we are born. The types of microorganisms vary based on diet and environment. For example, babies who drink breast milk will be exposed to different microorganisms than babies who drink formula. Similarly, adolescents and adults who eat diets high in sugar and fat will have different types of microorganisms in their gut compared with those who eat diets high in fruits and vegetables.
Since we are all unique in the specifics of our diet and immediate surroundings, the composition of the microorganisms that inhabit our gut differs from person to person. There are a range of species and relative amounts of microorganisms in our gut that keep us healthy. Outside of that range, though, is the possibility for sickness. Recent studies have linked changes in gut microorganisms to diseases such as
diabetes,
depression,
arthritis,
inflammatory bowel disease,
and
others.
Given that an imbalance in the microorganisms of the digestive tract can cause disease, it is important to be able to identify specific microorganisms inhabiting the gut of an individual person. Right now, clinicians do this by analyzing stool samples for specific microorganisms known to cause disease. For example, a patient complaining of stomach pain could be tested for the presence of the bacteria
Helicobacter pylori,
which can cause stomach ulcers.
However, this type of analysis provides an incomplete picture of the microorganisms in the gut. Determining the presence of a certain bacteria may be enough information to treat infection in the case of H. pylori, but the relationship between the gut and our overall health is more complex. For other types of infections, it might also be important to know how fast the bacteria is growing or how the growth of bacteria changes during disease. This is one application of a single-cell synthetic oscillator named the
Repressilator 2.0,
which was developed by Dr. Riglar’s collaborators, Dr. Laurent Potvin-Trottier, Prof. Johan Paulsson and others. Dr. Riglar and colleagues in Prof. Silver’s laboratory used the Repressilator 2.0 to track bacterial growth and demonstrate that it is able to continue to act robustly within the gut of a living animal.
Developing Synthetic Oscillators
The word “oscillation” means to go back and forth at a regular speed. One example of oscillation that you may be familiar with is a
pendulum,
which swings back and forth in a series of movements that repeat in cycles over time.
There are many types of oscillators in our everyday lives. The rotation of the moon around the Earth or the Earth around the sun can be thought of as natural oscillation patterns because they occur in regular cycles over time. In technology, electronic oscillators use oscillating electrical signals to convert one type of electrical current to another and to regulate time on a clock.
Within our bodies there are innate timing devices, called biological clocks, that display an internal oscillation of about 24 hours. Biological clocks produce
circadian rhythms
and regulate their timing. One example of a circadian rhythm is the sleep-wake cycle. The sleep-wake cycle, like other biological clocks in the body, is composed of specific genes and proteins whose levels oscillate.
The type of oscillator that Dr. Riglar and colleagues (Dr. Richmond, Dr. Potvin-Trottier, Mr. Verdegaal, Dr. Naydich, Dr. Bakshi, Dr. Leoncini, Ms. Lyon, Prof. Paulsson and Prof. Silver) use in their research is called a synthetic gene oscillator. The synthetic gene oscillator is an invention that has come out of the field of
synthetic biology.
Synthetic biology can be thought of as a form of engineering which uses the principles of
electrical
or
mechanical engineering
to build
circuits.
Rather than using wires, though, synthetic biology uses DNA and proteins to create living circuits.
The original version of the repressilator was developed by
Drs. Michael Elowitz and Stanislas Leibler in 2000.
It used three bacterial proteins that were related because the presence of each protein causes the repression of one of the other proteins. At the beginning of the cycle, protein A is expressed, meaning that the expression of protein B is inhibited. Over time, the amount of protein A will become reduced, allowing for the expression of protein B. The expression of protein B inhibits protein C until it too becomes reduced, allowing for the expression of protein A again. The expression of protein C inhibits protein A until it becomes diluted, and protein A is expressed again. Now we’re back at the beginning. From this example, you can see how the synthetic oscillator oscillates between three different settings in an ongoing cycle that repeats over and over again.
The researchers engineered the first synthetic gene oscillator using a living circuit of the three bacterial genes for the proteins involved. The genes were connected into a circular piece of genetic material known as a
plasmid.
The Repressilator 2.0
Dr. Riglar and his colleagues wanted to use a living circuit to better understand growth patterns in bacteria. Dr. Potvin-Trottier, Prof. Paulsson and colleagues had previously developed the Repressilator 2.0 circuit, building on the work of Elowitz and Leibler and others
(Potvin-Trottier et al. 2016),
which also added fluorescent markers to each of the bacterial genes in the circuit. This way, each part of the cycle of the oscillation could be identified by a specific color, as shown below. Through this work, Dr. Potvin-Trottier, Prof. Paulsson and colleagues were able to develop the oscillator so that it took the same number of bacterial generations to complete a full cycle in all the cells across a population.
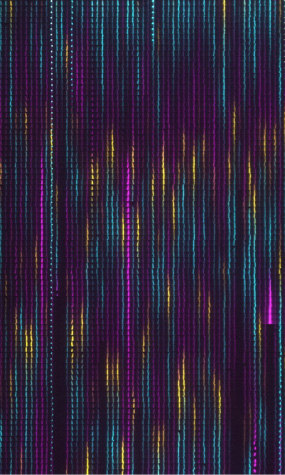
Figure 1. This visual schematic tracks the repressilator cycle through individual bacterial samples over time: the expression of each of the repressor proteins is represented by a different fluorescent color.
[Credit: : Paulsson Lab and Wyss Institute at Harvard University]
In order to test the Repressilator 2.0’s ability to measure bacterial growth, Dr. Riglar and colleagues inserted the repressilator plasmid into single bacteria that they plated on a petri dish. Once the starting bacteria had grown into colonies, the researchers took images and saw rings of the fluorescent markers, which indicate the growth of the bacteria.
The researchers were able to do this because bacterial colonies grow radially outward from the center of the colony. The bacteria at the outer edge of the colony are constantly dividing and making the colony bigger. In fact, the bacteria in the colonies in Figure 2 include about 40 generations. The result is that at any given radius the bacteria present in the ring around the colony have all undergone the same number of divisions. The bacterial colonies develop rings of fluorescence as they grow (Figure 2).
A
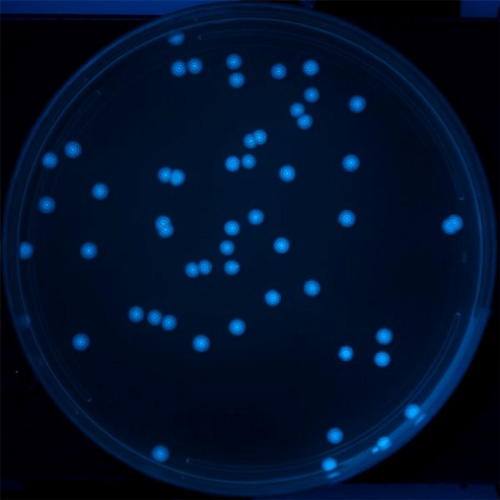
B

Figure 2. Image of colonies of bacteria containing the repressilator circuit on A. a petri dish and B. close-up. The bacterial colonies develop rings of fluorescence as they grow.
[Credit: Silver Lab and Wyss Institute at Harvard University]
The researchers noticed that the position of the fluorescent rings changed depending on the stage of the repressilator cycle of the starting bacterium. This allowed Dr. Richmond to write a computer program to identify the rings within each colony as a signature that indicated the stage of the repressilator of a given starting bacterium. The researchers called this technology RINGS, which stands for Repressilator-based INference of Growth at Single-cell level. By measuring the shift in the oscillator between two time points, they were able to calculate how much growth had happened during a given time interval.

Figure 3. The position of the fluorescent rings changes depending on what stage of the repressilator cycle the starting bacterium is in. This gif shows examples of colonies started by bacteria in different repressilator phases.
[Credit: Silver Lab and Wyss Institute at Harvard University]
Once the RINGS system had been validated in the petri dish, Dr. Riglar and his colleagues wanted to know whether the system would also work in living animals. To do this, they inserted the oscillator into a type of E. coli bacteria commonly found in the digestive tract of mice. They then used a syringe to inject a specific amount of bacteria into the mouths of the experimental mice so the bacteria would go straight
to the gut. They collected stool samples from the mice and analyzed the samples for the presence of the engineered plasmids and the stage of the repressilator.
Over the course of several experiments, the researchers were able to determine that the RINGS system was effective at identifying bacterial growth over time in mice for up to several days. “This was really exciting,” remarked Dr. Riglar. “Now that we’ve shown that this system works, we can use it to tell us lots of different types of information about the microorganisms in the gut.”
Next, mice were given a compound that causes inflammation in the gut followed by repressilator-loaded bacteria. The RINGS system showed that the bacteria from mice with inflammation had repressilators in a wider range of phases compared to bacteria from control mice. This suggests that inflammation changes the gut environment, resulting in different growth patterns in the bacteria. It is possible that these changes may cause imbalances in the microorganisms that naturally occur in the gut. This area of research is ongoing in Dr. Riglar’s laboratory.
Dr. Riglar and his team would also like to know if the difference in growth rates in bacteria depend on the area of the gut where they are found. For example, they hope to determine whether bacteria grow faster in the stomach, or the small intestine, or the colon. Eventually, many years from now, Dr. Riglar hopes to develop a tool that allows for the analysis of bacteria in the gut for diagnostic purposes. “We are just now learning about all the connections between the bacteria in our gut and our overall health. I hope our research can help identify some of the bacteria that might be causing us the most problems.”
Dr. David Riglar is Sir Henry Dale Research Fellow in the Department of Infectious Diseases at Imperial College in London. The work described here was completed during his time as a post-doctoral fellow at Harvard Medical School and the Wyss Institute for Biologically Inspired Engineering in the laboratory of Dr. Pamela Silver. The current focus of Dr. Riglar’s research is understanding how microorganisms in the gut respond to inflammation. When not in the laboratory, Dr. Riglar enjoys cooking and spending time with his family.
For More Information:
- Riglar, D. et al. 2019. “Bacterial variability in the mammalian gut captured by a single-cell synthetic oscillator,” Nature Communications, 10(1): 1-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6789134/
- Brownell, L. “A reliable clock for your microbiome.” 11 Oct 2019. https://wyss.harvard.edu/news/a-reliable-clock-for-your-microbiome/
- Potvin-Trottier, L., Lord, N., Vinnicombe, G. et al.Synchronous long-term oscillations in a synthetic gene circuit. Nature 538, 514–517 (2016) doi:10.1038/nature19841 https://www.ncbi.nlm.nih.gov/pubmed/27732583
- Elowitz, M., Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000) doi:10.1038/35002125. https://www.ncbi.nlm.nih.gov/pubmed/10659856
To Learn More:
- Riglar Lab. riglarlab.com
- Silver Lab. https://silver.med.harvard.edu/
- The Microbiome and Disease. https://learn.genetics.utah.edu/content/microbiome/disease/
- Gut Bacteria in Health and Disease. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983973/
- The Gastrointestinal Tract. https://www.youtube.com/watch?v=1SHszPMSRQA&vl=en-US
- The GI System. https://www.le.ac.uk/pathology/teach/va/anatomy/case6/frmst6.html
- Synthetic Biology. https://www.genome.gov/about-genomics/policy-issues/Synthetic-Biology
- What is Synthetic/Engineering Biology? https://ebrc.org/what-is-synbio/
- Your Digestive System and How it Works. https://www.niddk.nih.gov/health-information/digestive-diseases/digestive-system-how-it-works
- Circadian Rhythms. https://www.nigms.nih.gov/education/pages/factsheet_circadianrhythms.aspx
- Introduction to the Human Gut Microbiota. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5433529/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |
|
|

Dr. David Riglar

Dr. Pamela Silver

Dr. David Richmond

Dr. Laurent Potvin-Trottier
Sign Up for our Monthly Announcement!
...or  subscribe to all of our stories! subscribe to all of our stories!

What A Year! is a project of the Massachusetts
Society for Medical Research.
|
|

