Highlights
Sickle cell disease is caused by a single mutation in a gene that encodes for the blood protein, hemoglobin, which transports oxygen throughout the body. This mutation significantly alters the shape of the hemoglobin protein and its ability to function. These researchers have developed a way to correct the genetic mutation using gene therapy, and then transplant the corrected stem cells back into the patient. Clinical trials for people with severe sickle cell disease are currently underway. These findings offer the possibility for a cure for sickle cell disease and other genetic diseases whose exact genetic mutation is known.
Did you know that the human
genome
contains 20-25 thousand genes? Genes are composed of deoxyribonucleic acids, or DNA, connected in specific sequences that provide the instructions for the formation of proteins. If there is a mistake in even one segment of this sequence, known as a
mutation,
the consequences can be very serious.
There are a whole range of
genetic disorders
caused by such mutations, including
sickle cell disease
(SCD). Recently, Dr. John Tisdale and colleagues from the National Institutes of Health have shown that it might be possible to cure genetic diseases such as SCD by correcting the mutations that cause them. The researchers hope this new technique might offer a cure for millions of people around the world living with genetic diseases.
Our DNA is “written” by just four molecules called
nucleotides:
guanine, adenine, thymine, and cytosine, or G, A, T, and C for short. In the parts of our DNA that provide the instructions for proteins, every three nucleotides indicate an
amino acid.
Proteins are made up of large strings of amino acids in the sequence described by the DNA.
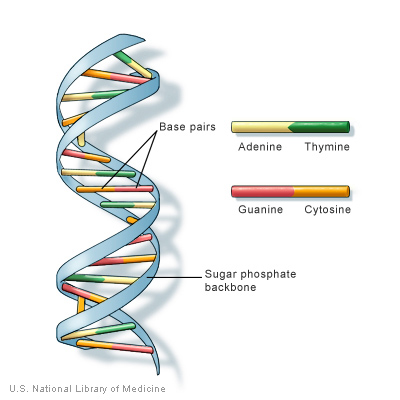
Figure 1. Structure of DNA
[Source: https://ghr.nlm.nih.gov/primer/basics/dna]
Hemoglobin and Sickle Cell Disease
The impact of a genetic mutation depends on where the mutation occurs in the DNA. Sometimes a mutation has no impact because it is not part of the instructions for a protein or the change does not affect protein structure. In the case of genetic diseases, though, the mutation has a large impact. In patients with SCD, a single nucleotide is affected in the gene that encodes for the blood protein
hemoglobin
on chromosome 11: the A in G-A-G becomes a T. This “spelling error” changes the amino acid produced from glu (glutamine) to val (valine). This significantly alters the shape of the hemoglobin protein and its ability to function.
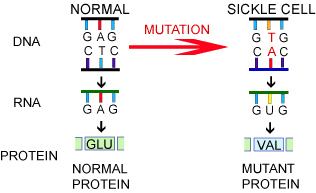
Figure 2. Mutation in Sickle Cell Disease
[Source: https://evolution.berkeley.edu/evolibrary/article/0_0_0/mutations_06]
Hemoglobin is produced in the
bone marrow
by cells that become
red blood cells
and is necessary for oxygen to be transported through the bloodstream. Hemoglobin plays an important role in maintaining the shape and structure of red blood cells.
In patients with SCD, the mutation in the hemoglobin gene causes the hemoglobin protein to be misshapen. As a result, the misshapen hemoglobin can carry less oxygen than normal through the bloodstream. In addition, the misshapen hemoglobin changes the shape of the red blood cells from round discs that travel easily through blood vessels to thin slivers that are more likely to get stuck and block blood vessels, especially small blood vessels called
capillaries.
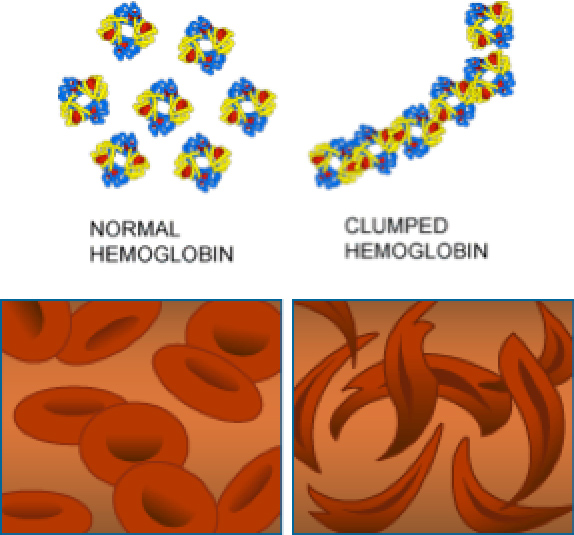
Figure 3. Healthy red blood cells and sickled red blood cells
[Source: https://evolution.berkeley.edu/evolibrary/article/0_0_0/mutations_06]
Clinicians who first identified SCD thought the abnormal red blood cells looked like a sickle, an agriculture tool with a rounded blade. As a result, this disease became known as sickle cell disease.

Figure 4. Picture of a sickle
[Source: https://www.reading.ac.uk/merl/research/interface/merl-sickle.aspx]
SCD is characterized by acute and chronic pain and a variety of complications affecting many organ systems. There are treatments available to help reduce pain and prevent complications, and it is possible to cure some patients with
bone marrow transplants.
However, only about 15% of patients with sickle cell disease have an appropriate
bone marrow match
that would make the transplant successful.
The Promise of Gene Therapy
Dr. John Tisdale first became aware of SCD during his medical school residency in the 1990s. Patients with SCD would come into the emergency room with severe pain, and there was nothing specific the doctors could do. Doctors could give patients pain medicine and intravenous (IV) fluids, but there was little they could do to prevent another episode in the future. Dr. Tisdale was motivated to try to develop better treatments for this disease.
Since SCD is a genetic disease with a known genetic mutation,
gene therapy
seemed like a promising technique to offer a cure. Gene therapy is a type of treatment that seeks to cure disease by correcting the genetic mutation(s) that cause it. The ultimate goal is to take the patient’s own
hematopoietic (blood-producing) stem cells,
correct the genetic mutation(s) using gene therapy, and then transplant the corrected stem cells back into the patient. In fact, Dr. Tisdale and his team have successfully cured several patients with SCD using gene therapy. Let’s explore how they got there.
For decades researchers had been trying to use parts of viruses as vehicles to deliver new genetic information to cells. Viruses are very good for this purpose because they function by inserting their own genetic material into the host cells they infect. Researchers strip the virus of the disease-carrying parts, leaving just a shell, and the researchers can insert whatever genetic material they choose. These virus-like vehicles are called
viral vectors.
The researchers knew they had to choose their viral vector carefully. Previous research had tried to use non-human viruses such as the
murine leukemia virus
in mice. In order to be most effective, though, the virus would have to be able to cause infection in humans as well. Dr. Tisdale and his colleagues turned to the
human immunodeficiency virus
(HIV), the virus that causes
AIDS,
because it is so effective at transferring genetic information to host cells.
“We removed all the parts of the virus that cause infection, leaving just the shell of the virus,” explained Dr. Tisdale. “Then we added the corrected genetic sequence on chromosome 11, the genetic sequence that produces hemoglobin.” The cells with the corrected gene are given back to the patient via an IV line and find their own way into the bone marrow.
In a typical experiment, Dr. Tisdale and colleagues put the stem cells in a flask and exposed them to the viral vector. After exposure, the researchers took the corrected stem cells and transplanted them into
mouse models
of SCD.
In order to prepare for the transplant, the mice needed to undergo a process called a
conditioning regimen.
As Dr. Tisdale described, “when you plant a garden, you have to pull out all the weeds before you can plant new seeds.” In other words, the animal’s own stem cells that produce misshapen blood cells must be destroyed. The researchers used
radiation
to prepare the bone marrow to receive new stem cells.
Once the bone marrow stem cells were transplanted, the researchers examined the blood of the experimental mice to see how effective the transplant was. Previous research had shown that 20% of the blood cells must be normal in order to correct SCD. This is because normal red blood cells last much longer than sickle-shaped red blood cells.
Dr. Tisdale and his team conducted many experiments like the one just described. They used different combinations of specialized proteins called
growth factors
to increase the effectiveness of the transplanted stem cells.
Eventually, the team found the right combination of factors to yield the highest amount of normal red blood cells in mouse models of SCD. Next, they conducted similar experiments in larger animals, like monkeys. Once there was enough evidence the gene therapy was safe and effective in large animal models, it was time to begin a
clinical trial,
testing in human patients with severe, life-threatening SCD.
Human Trials
Given the experimental nature of this therapy, patients were selected based on the severity of their disease. Only patients with severe SCD were allowed to participate, and all patients participated in the clinical trial on a volunteer basis.
For patients selected for the study, the first step was to give a sample of their bone marrow stem cells. The researchers exposed each patient’s stem cells to the viral vector to make sure the stem cells could receive the corrected genetic information. The patient’s stem cells were then frozen while the patient was prepared for conditioning.
Conditioning consisted of four days of high-dose
chemotherapy.
This process can be very painful, and it comes with many side effects like hair-loss, nausea, and vomiting. However, for these patients, the possibility of life without SCD was worth the side effects.
Once the patient was ready to receive the stem cell transplant, the corrected stem cells were unfrozen and tested again to make sure they were working. The patient was given the corrected stem cells through an IV line in the arm, and the cells made their way to the bone marrow. The patient usually spent about three weeks recovering in the hospital as the corrected stem cells entered the patient’s bone marrow and the bone marrow started producing normal blood cells.
So far, the human trials have been very successful. “For patients with this disease, the gene therapy can be transformative,” explained Dr. Tisdale. “They have been living with a chronic disease that limits their ability to do many things, and if affects the whole family too. Life without that is extraordinary, it is life-changing.”
Future Work
Although the results of gene therapy in patients with SCD have been very promising, there is still more work to be done for the treatment to become available to more people with SCD. One major area of study for Dr. Tisdale and his team is developing better methods of conditioning that are less difficult for the patient to tolerate. Chemotherapy causes many side effects because it is not specifically targeting bone marrow stem cells—it kills any cells that are dividing fast. This means that other cells that divide quickly, like blood cells, hair cells and intestinal cells, are also affected.
Future methods would try to target bone marrow cells specifically to avoid such side effects. One way to do this is by developing
antibodies
that would target just the bone marrow stem cells. This would allow for conditioning of the bone marrow without affecting other parts of the body and causing so many side effects.
Another area of future work is applying the same gene therapy techniques to related diseases such as severe
combined immunodeficiency disease
(SCID). Children born with SCID appear normal at birth. However, genetic mutations in the genes that encode for cells of the
immune system,
the cells that fight disease and infection, make children with SCID highly susceptible to infection. The condition is fatal in the first year or two of life unless the patient receives life-saving treatment through stem cell transplants or gene therapy. Dr. Tisdale and colleagues hope that the technique they have developed for SCD might also offer treatments that are more broadly available with fewer side effects to patients with SCID.
Dr. John Tisdale is Senior Investigator in Molecular and Clinical
Hematology
at the National Institutes of Health. His research focuses on treatments for sickle cell disease and related genetic blood diseases. Dr. Tisdale and his colleagues at the National Institutes of Health have a rock band called the Free Rock and Rollers where they play at different events and fundraisers. When not in the laboratory, Dr. Tisdale is playing music with his colleagues and friends.
For More Information:
- Leonard, A. and J. Tisdale. 2018. “Stem cell transplantation in sickle cell disease: therapeutic potential and challenges faced.” Expert Review of Hematology, 11(7): 547-65.
- Could gene therapy cure sickle cell anemia? https://www.cbsnews.com/news/could-gene-therapy-cure-sickle-cell-anemia-60-minutes-2019-12-29/
To Learn More:
- NIH researchers create new viral vector for improved gene therapy in sickle cell disease. https://www.nih.gov/news-events/news-releases/nih-researchers-create-new-viral-vector-improved-gene-therapy-sickle-cell-disease
- Sickle cell patient’s recovery after gene therapy heightens hopes for a cure. https://www.nhlbi.nih.gov/news/2019/sickle-cell-patients-recovery-after-gene-therapy-heightens-hopes-cure
- About Sickle Cell Disease. https://www.genome.gov/Genetic-Disorders/Sickle-Cell-Disease
- Sickle Cell Disease. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease
- Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/sicklecell/facts.html
- What is Sickle Cell Disease? https://www.cdc.gov/ncbddd/sicklecell/facts.html
- American Society of Hematology. https://www.hematology.org/education/patients/anemia/sickle-cell-disease
- Sickle Cell Disease Coalition. http://www.scdcoalition.org/
Written by Rebecca Kranz with Andrea Gwosdow, PhD at www.gwosdow.com
HOME | ABOUT | ARCHIVES | TEACHERS | LINKS | CONTACT
All content on this site is © Massachusetts
Society for Medical Research or others. Please read our copyright
statement — it is important. |

